Next generation advanced medicines are being introduced through improvements in biopharmaceutical innovation, however, the duality of the industry is often overlooked. While the industry is credited with developing life-saving therapies such as advanced cell and gene therapies, it is also producing more greenhouse gas emissions than the automotive industry as an unintended consequence of its innovation and manufacturing processes. Additionally, supply chain challenges associated with decentralized manufacturing, temperature-sensitive products, and the prevalent usage of single-use consumables in around 85% of bioprocessing, (particularly in upstream processes like cell culture[1], cell separation, and harvest), can make change seem insurmountable. But if we take a step back and narrow our focus, transformation is readily achievable.
Cell culture media with its plastic packaging and single-use vessels for processing is among the various factors that contribute to the eco-unfriendly nature of life sciences. As a critical raw material for both research and therapy development, the stage is set for a much-needed paradigm shift.

To deliver a catalyst for that change, Krakatoa, (manufactured by Stoic Bio and distributed by Nucleus Biologics) leverages the in-depth understanding of liquid media manufacturing from Nucleus Biologics, the leading provider of small lot custom media. There were two primary objectives: to accelerate therapy development by offering the type of custom media required to enhance and optimize processes and efficacy, and provide this optimized media in a more environmentally sustainable manner compared to traditional cell culture methods. It accomplishes this as the world’s first benchtop media maker, solubilizing and dispensing media on-demand, at point of use. Using proprietary, recyclable pods containing powdered media, the machine utilizes ultra-pure water to solubilize and dispense media into a 500 mL reusable glass bottle – all completed within the device at the push of a button. With Krakatoa, Nucleus Biologics is providing laboratories with a simplified pathway to quality custom media that enables faster iteration, in one of the greenest forms of cell culture media currently available in the market.
Curing People Faster
As the biopharmaceutical industry endeavors to cure diseases in a world constrained by its current supply chain, developers must carefully scrutinize each step and component in their processes, including the attributes of raw materials. As a result, media composition has become a prominent topic with the current standard relying on proprietary, off the shelf products that offer little to no information about their composition. This lack of knowledge makes it difficult for developers to troubleshoot and optimize critical quality attributes and process outputs. In addition, as we focus attention to the variable nature of biologics and the biological effects linked to individual cell culture components, there is an increasing requirement for highly customized media, emphasizing the need to democratize cell media composition.
Formula ownership and rapid iteration of custom media can accelerate therapy development and reduce time to scale up—the ultimate qualifying benefits provided by Krakatoa. With a minimum volume of 500 mL, users can move faster in the early stages of development and at lower cost before ramping up to bioreactor scale as they move downstream. However, the journey from bench to bioreactor is marked by another significant factor: the media that arrives and is subsequently solubilized with Krakatoa in the laboratory is of great potency. Transparency in media formulation is highly significant in this context. Studies have shown[4] that there are significant changes in amino acid concentration in human serum under different storage and pre-storage conditions. Other researchers[4] have also found significantly increased levels of tryptophan oxidation products, as well as riboflavin degradants in culture media due to light exposure post solubilization. Such risks affect lot-to-lot consistency and overall efficacy of the media of which end-users will never be able to ascertain from a COA or otherwise. But there are more commonplace scenarios as well—have you ever had a temperature-sensitive media arrive late? A highly sensitive reagent package left out in the wrong place, only for you to question its integrity and stability? By manufacturing powdered media and shipping it in pods, these risk factors are mitigated, and by solubilizing at point-of-use, this provides greater assurance of the media’s potency.
Curing the Planet
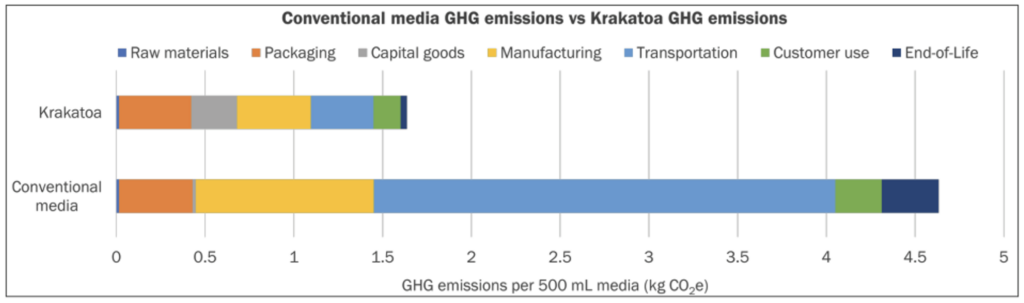
The inherent advantages and greatest impact of using pod-based media are also realized with their reduced impact on the environment when systematically assessed over the product lifecycle. In a Life Cycle Assessment (LCA) conducted by VitalMetrics in collaboration with Nucleus Biologics, the two companies sought to compare the pod-based media manufacturing system to that of conventional liquid cell culture media shipped in Polyethylene terephthalate (PET) plastic bottles. The results were staggering— in a bottle-to-pod comparison, for each 500 mL unit of media produced, Krakatoa technology reduces greenhouse gas emissions by 65%. A significant factor that contributes to this reduction is the composition of the pods, which are comprised of 96% biodegradable plastic. Stoic Bio has also established a closed-loop supply chain having instituted a send-back program to recycle used pods.
Shipping powdered media pods not only ensures potent media as mentioned in the previous section, but also has a larger advantage in reducing cold chain requirements. The LCA estimates that, “50% of all conventional media requires icepacks for domestic travel, and 100% for international transport.” With most formulations contained in media pods transported and stored at ambient temperature, cold chain requirements are vastly reduced compared to liquid media. Additionally, media is primarily shipped by air where weight is directly related to fuel usage, and each pod weighs 80% less than a 500 mL bottle of liquid cell culture media. The benefit and reduced environmental impact of pod-based media will be further substantiated as volumes scale with next generation technologies.
Changing the Paradigm
The issue of sustainability remains an emerging topic of discussion and requires innovative technologies, advocacy, and radical change to bring the issue to the forefront of the industry and lessen its impact on the environment. Krakatoa is an example of such a technology that not only drastically reduces a lab’s impact but also provides numerous competitive advantages, making it an essential tool to facilitate change.
Other industry focuses place greater emphasis on media efficacy and composition, and have exposed the issue of single source supply as a critical constraint in the supply chain. A company’s supply chain resiliency hinges on its ability to source the components it needs for development and manufacturing, and the COVID-19 pandemic underscored the importance of having a supply chain that can cope with supply disruptions. Moreover, when working with a single supplier for proprietary products without any ownership over the formula, the ability to source is significantly hampered when supply issues occur with that supplier. With custom media used in Krakatoa, customers retain ownership of their intellectual property and are not constrained by single source supply.
To further facilitate change, future iterations and additions to the Krakatoa product family will include high-throughput systems for bioprocessing use, for large scale, point-of-use media.


